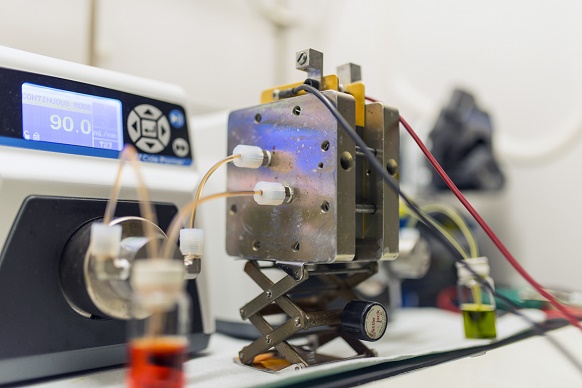
Harvard University researchers say they have identified a new class of high-performing organic molecules, inspired by vitamin B2, that can safely store electricity from intermittent energy sources – such as wind and solar power – in large batteries.
According to a news release from the university’s John A. Paulson School of Engineering and Applied Sciences (SEAS), the development builds on previous work in which the team developed a high-capacity flow battery that stored energy in organic molecules called quinones and a food additive called ferrocyanide. The researchers say that advancement was a game-changer because it delivered high-performance, non-flammable, non-toxic, non-corrosive and low-cost chemicals that could enable large-scale, inexpensive electricity storage.
The university says although the versatile quinones show great promise for flow batteries, Harvard researchers continued to explore other organic molecules in pursuit of even better performance. However, the researchers say finding that same versatility in other organic systems has been challenging.
“Now, after considering about a million different quinones, we have developed a new class of battery electrolyte material that expands the possibilities of what we can do,” says Kaixiang Lin, a Ph.D. student at Harvard and first author of the researchers’ paper on the topic. “Its simple synthesis means it should be manufacturable on a large scale at a very low cost – which is an important goal of this project.”
Flow batteries store energy in solutions in external tanks – and the bigger the tanks, the more energy they store, Harvard explains.
In 2014, Michael J. Aziz, the Gene and Tracy Sykes professor of materials and energy technologies at SEAS; Roy Gordon, the Thomas Dudley Cabot professor of chemistry and professor of materials science; Alán Aspuru-Guzik, professor of chemistry; and their team at Harvard replaced metal ions used as conventional battery electrolyte materials in acidic electrolytes with quinones, which store energy in plants and animals. In 2015, they developed a quinone that could work in alkaline solutions alongside a common food additive.
In this most recent research, the team found inspiration in vitamin B2, which helps to store energy from food in the body. According to the researchers, the key difference between B2 and quinones is that nitrogen atoms, instead of oxygen atoms, are involved in picking up and giving off electrons.
“With only a couple of tweaks to the original B2 molecule, this new group of molecules becomes a good candidate for alkaline flow batteries,” says Aziz. “They have high stability and solubility and provide high battery voltage and storage capacity. Because vitamins are remarkably easy to make, this molecule could be manufactured on a large scale at a very low cost.”
Harvard says the team will continue to explore quinones, as well as this new universe of molecules, in pursuit of a high-performing, long-lasting and inexpensive flow battery.
The university says its Office of Technology Development has been working closely with the research team to navigate the shifting complexities of the energy storage market and build relationships with companies well positioned to commercialize the new chemistries.
The paper, published here in Nature Energy, was authored by Lin, Aziz, Gordon, Aspuru-Guzik, Rafael Gómez-Bombarelli, Eugene S. Beh, Liuchuan Tong, Qing Chen and Alvaro Valle.
The research was supported by the U.S. Department of Energy’s Advanced Research Projects Agency-Energy, the Massachusetts Clean Energy Technology Center and the National Science Foundation.
Photo courtesy of Kaixiang Lin/Harvard University